

In early March, snowbanks and frosty mornings remind us it’s still winter–but by month’s end longer days and warmer winds prevail. On March 20, the vernal equinox marks the arrival of spring in the Northern Hemisphere. Here are some signs of spring to look for in the natural world to tide you over until warmer weather truly arrives.
By Julia Pupko
While hiking through dense mixed or coniferous forests around dawn or dusk, you may hear a clear-sounding too-too-too-too, almost as if someone is perched in a tree playing the same note over and over again on a recorder. Someone is perched in a tree, but they are not playing a recorder. This is actually the call of Vermont’s smallest owl, the Northern Saw-whet Owl (Aegolius acadicus). Like many other raptor species, female Northern Saw-whet Owls are larger than males. Males are 18 to 20 cm in length and weigh around 75 grams (roughly that of an American Robin), and females are 20 to 21.5 cm long, weighing around 100 grams. While there have been many studies on the subject, there is still no consensus as to why female raptors are larger than their male counterparts. Hypotheses suggest the size difference reduces hunting competition between mates, or that larger size gives females an advantage over defending her territory from other females and aggressive males. Another hypothesis suggests that the female’s larger size helps her better defend her nests from other raptors, raccoons, etc. Females usually spend more time incubating eggs than males do, so being larger may help them defend the nest more effectively. Males typically provide food, and having a smaller body size may allow them to more effectively hunt smaller prey, which are typically more numerous.
Regardless of the reason for the size difference between males and females, Northern Saw-whets are entering their breeding season this month, and will breed from March to July. Males typically arrive to breeding habitats first; many Northern Saw-whet Owls migrate south for the winter, but some individuals are likely non-migratory and remain in their breeding range year-round. To attract a mate, males begin to sing, too-tooing away from sunset to sunrise. Males sometimes start singing as early as January, and their songs can be heard up to a half mile away.
When a female is interested in what she hears, she will respond to the male with a high-pitched call or series of whistles. The male will then circle his prospective mate before landing beside her and presenting her with a gift of a prey item, such as a mouse. After this, they may preen each other to form a bond. Nest initiation typically occurs in March. Females will choose the nest site, laying four to seven eggs in a tree cavity or abandoned woodpecker hole that is between two and 12 meters above the ground. Northern Saw-whet Owls generally occupy boreal coniferous forests in Vermont, but dead deciduous trees seem to be preferred for nesting if they are accessible.
Females incubate the eggs for 26 to 28 days before they hatch. The female is primarily in charge of incubating and protecting the young, while the male hunts to provide food for his mate and chicks. Northern Saw-whets hunt at night, preying on small mammals, including voles, shrews, mice, and lemmings, along with the occasional squirrel or chipmunk. They like hunting along forest edges, roosting in a tree or shrub while they listen for prey. Northern Saw-whet Owls will also opportunistically prey on insects and small birds.
Roughly 18 days after the chicks hatch, females leave the nest and either assist the male with hunting or will leave the area in search of another mate. Chicks leave the nest at four to five weeks of age. They can fly at this point, but the male remains with them for another month, continuing to feed them. At six to eight weeks of age, the young venture off on their own.
Northern Saw-whet Owls are typically very elusive, roosting in thick coniferous vegetation during the day. Listening for their songs during the breeding season can be a great way to “observe” them in a sense. If you hear one, make sure to record the song if you can and submit your observation to Vermont eBird!
By Julia Pupko
Have you ever thought about checking your bird feeder in the middle of the night? If you have a feeder and your house is surrounded by mature forest habitats, it’s worth shining a late-night light on your feeder. You may just see a Flying Squirrel!
In Vermont, we have two species of Flying Squirrel: the Northern Flying Squirrel (Glaucomys sabrinus) and the Southern Flying Squirrel (Glaucomys volans). They are very similar in appearance, but can be distinguished by size (Southern Flying Squirrels are smaller) and that the belly hair of the Southern Flying Squirrel is white all the way to the base of each hair, while Northern Flying Squirrels have a dark undercoat on their stomachs. Northern Flying Squirrels prefer mature boreal coniferous and mixed coniferous forests, but can also be found in deciduous forests, both in boreal zones and at lower elevations. They eat mostly buds, berries, insects, lichens, and fungi, even during winter, but will occasionally take advantage of masts and available seed.
Northern Flying Squirrels begin their courtship in March, and their mating season lasts until May. Females are entirely responsible for caring for the young, and do not remain with the male after mating. The female gives birth to a litter of two to four young, a month to a month and a half after mating. Cavities in snags and trees are used for nests, and Northern Flying Squirrels sometimes share nests, living in groups of up to eight juveniles and adults. It is likely that females only have one litter per year, but little is known about their reproductive biology. The mother Northern Flying Squirrel weans the young when they are around two months old. Young will usually remain with their mother for another month before becoming independent.
Climate change and habitat loss are both stressors for the Northern Flying Squirrel. The species is relatively versatile in terms of their habitat use, but seem to need mature forests that host snags and decomposing logs to provide nesting sites and hosts for fungal foods. As a result, Northern Flying Squirrels are sensitive to loss of these forests, both from introduced pests, such as the Hemlock Woolly Adelgid, and from human land alterations, such as logging, which has strained populations historically. Additionally, Northern Flying Squirrels seem to thrive in forests that have relatively cold winters, while Southern Flying Squirrels have a lower tolerance for cold temperatures. In the southern Appalachian Mountains, Southern Flying Squirrels are mostly found at lower and mid-elevations, while Northern Flying Squirrels are restricted to boreal zones. In New England, Northern Flying Squirrels can be found at a greater range of elevations, while Southern Flying Squirrels are restricted to lower-elevation valleys that have milder winters.
While these species do interact to a minimal extent where their habitats overlap, many populations remain relatively separate, which may be for the best: Northern Flying Squirrels seem to be highly susceptible to a nematode parasite, Strongyloides robustus, that is often carried non-fatally by Southern Flying Squirrels. This parasite thrives in warmer climates, which are preferred by Southern Flying Squirrels as well, meaning that climate change combined with habitat restrictions may increase transmission risk of these parasites to Northern Flying Squirrel populations, especially in the southern edges of their range. Even though this nematode does not always kill Northern Flying Squirrels, it usually greatly reduces their fitness.
By Spencer Hardy
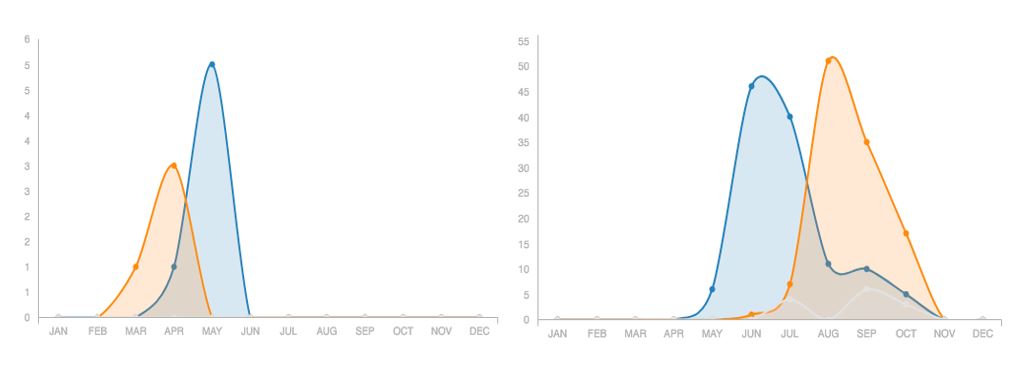
As we approach the end of winter, it’s worth pausing to think about how tiny, hyperactive insects like bees are able to survive extended sub-zero periods and many months without nectar. It turns out there are lots of different ways! The Honey Bee (Apis melifera) is the only species active during winter, with the colony balled together to keep everyone warm. Most of our native bees are in the ground right now, either in their own nest or an old rodent burrow. Other species, however, choose to ride out the winter in hollow stems or even rotten logs. To complicate the story, some bees are in their adult form right now, while others are pupa and won’t get their wings until they are ready to emerge over the summer. Logically, bees that are pupa right now will need to mate before they can reproduce over the summer, but some of the genera that hibernate as adults mate in the fall and males won’t exist again for another six or so months. The two graphs below illustrate these different natural history strategies (and the power of iNaturalist). In the case of the Frigid Mining Bee, males come out just before the females and die while the females are still provisioning the next generation. Striped Sweat Bees, on the other hand, mate in the fall and only the females survive the winter.
By Julia Pupko
Serviceberries (Amelanchier sp.) are one of the first species to begin unfurling their leaves and opening their flowers each spring. Also called Shadbush, they are known for flowering in April around the time when Shad fish begin their upstream runs. The name “Serviceberry” comes from colonial times, when colonizers used the flowering times of the Serviceberry as a sign that the soil had thawed enough for them to begin burying their dead.
Despite the morbid common name, Serviceberries are truly beautiful plants. Their flowers are white with yellow centers, erupting with vigor across the whole tree. Their bark is gray with dark gray stripes, and usually smooth unless a tree reaches maximum size. Serviceberries typically remain small and relatively shrubby, but can grow to be upwards of 30 feet. While you may not see their flowers until early next month, you will probably not need to wait that long before you see signs of life from our Serviceberries. Given a few days of warm weather, their leaves will begin to unfurl. If you see a Serviceberry leafing out or flowering, be sure to report it to iNaturalist.
By Pete Kerby-Miller
Equipped with snowshoes and some necessary stoicism, I waded through deep snow and thick brush favored by moose. I wasn’t looking for moose particularly, but rather their top predator: winter ticks. Yes, these towering, 1,000 pound creatures are regularly brought down by arthropods no bigger than the last knuckle of your pinky finger. It’s a salient reminder that parasites, as E. O. Wilson put it, are “predators that eat prey in units less than one.” With up to 75,000 ticks found on a single moose though, these parasites can be quite deadly.
Moose populations have declined in recent years as a result of winter tick predation, but they are unlikely to crash entirely. With lower moose numbers, it becomes harder for the larval ticks to come across their moose hosts and tick numbers will eventually decline. It’s possible that the recent outbreaks of ticks in New England are a consequence of 19th century extirpation of wolves from the region. Without predation from wolves, modern moose populations might have grown until they were dense enough to be particularly vulnerable to ticks passing from nearby individuals. If food doesn’t limit an herbivore population, predation does.
The recent boom in winter ticks is also linked to warmer spring temperatures. Female ticks begin to fall off their hosts in March. If they fall on a mild spring’s bare ground, they’ll lay eggs, but if winter snow remains, many will freeze and die. A cold spring might reduce tick numbers temporarily, but reports from 112 years (and almost 1ºC global temperature increase) ago still describe winter ticks as “a greater enemy of the moose than wolves, bears, and cougars.” Today, state wildlife managers manage with winter ticks in mind. Lacking substantive predation from wolves, bears, and cougars, they hope that moose hunts in the densest populations can make it harder for ticks to spread.
Look for winter ticks in March snowbanks. The dark adults stand out on light backgrounds. Moose beds might also be flecked with telltale blood spots from feeding ticks. Whenever you look though, drive carefully–vehicle collisions are second only to 15 mm long parasites as predators to moose.
By Julia Pupko
As we are well aware, climate change has many impacts. In New England, climate change has been drastically impacting our winters in particular. The winter season is warming three times faster than our summers, producing drastic temperature fluctuations throughout the season, a more inconsistent snowpack which melts earlier, and earlier arrival of spring weather. Climate change-induced alterations to New England’s winters has a plethora of implications for our ecological systems, economy, and human health. I am going to focus on just one of many impacts of climate change, which is that on aquatic organisms.
There are several ways in which climate change has been affecting the aquatic organisms of Vermont and New England at large. The snowpack depth, ice thickness, and length of time with snow and ice cover are decreasing. Snowpack and ice are melting earlier in the spring and rain-on-snow events are increasing, particularly towards the end of winter.
Since ice and snow are melting earlier, and there are now rain events during parts of the winter where precipitation has historically been in the form of snow, peak stream flows during winter and spring are occurring earlier in the year. This impacts fish and other aquatic species that have evolved their life cycles around these peak flows, including migrations, spawning, and development of young. Additionally, earlier melt combined with more rain is changing the magnitude of stream and river flow. The influx of water can increase scrubbing along the bottom of waterways, which is when water removes sediment, stones, and other material from the bottom. Fish eggs and macroinvertebrates can get swept away too, taking a toll on populations. Scrubbing can also reduce habitat for aquatic organisms and their young, further limiting their ability to survive.
Ice and snowmelt occurring earlier in the year is shortening the amount of time that water bodies are frozen, and warmer winter temperatures are reducing the thickness of ice and snow layers over water bodies. A layer of ice and snow over water is important for aquatic organisms, as it provides insulation from fluctuations in air temperature. Additionally, the layer of ice and snow changes the light conditions of the water, which is important for the development of some fish species’ eggs.
Climate change has further implications for aquatic ecosystems outside of winter, as precipitation, flow, and evaporation are changing during all seasons. It’s not clear what the full implications of climate change will be for these systems, making it all the more important for continued research and for communities and leaders in Vermont and beyond to actively work to reduce emissions and mitigate climate change.
By Kent McFarland
The Wood Frogs behind my house are mostly frozen. Four or five months ago, when ice began to form inside their bodies, glucose levels flooded their blood rising by as much as 200-fold in just eight hours. It acts as an antifreeze and preserves tissues and organs through the long winter. But ice forms throughout their abdominal cavity and encases all internal organs. Large, flat ice crystals run between the layers of skin and muscle, and their frozen lenses make their eyes appear white. Blood stops flowing and as much as 65% of the frog’s total body water becomes ice. Breathing, heart beat, and muscle movements all stop. The frozen frog is in a state of suspended animation until spring thaw.
Wood Frogs are aroused when spring rains and melting snow begin to seep into the ground. They thaw from their frozen state, start up internal systems, and make the annual trek to breeding pools. Remarkably adapted to the cold, it is not unusual to find individuals scampering across old snow or swimming in water amidst ice.
Wood Frogs are explosive breeders, and most mating in a given pool takes place over just a few days. The loud duck-like calls of males are often a key to finding these pools. Females often deposit their gelatinous egg masses communally. The center of an egg mass may be up to 5°F warmer than the surrounding water, speeding development. Eggs often become covered by symbiotic algae (Oophilia ambystomatis) that enhance the oxygen supply to developing embryos in exchange for nutrients and carbon dioxide.
By mid- to late-summer nearly all juvenile frogs have left the pool as it dries up. Over 70% of these will succumb to predation before reaching adulthood. The rest will be frozen in time until spring comes again.
I love these short informative articles.
Thanks much for them.
Reading these makes for a peaceful little joy over morning coffee, and sparks desire to tromp out and marvel at every last thing around us. We’re blessed to be in this wonderland, Vermont. Thank you.
Thank You for this information, always good to hear from you.
Hi,
Have been on VPA’s listserv and no postings for Vt.
I’m looking for info re. VP activity, movement of sals, etc. specifically in Upper Valley this time of year.
Can’t find anything.
Can you help?!
Thanks,
Suzanne
Hi Suzanne – Check out the Hartford Salamander Team’s website! They are doing a super job of informing people about amphibian movement and how to help: https://hartfordsalamanderteam.org/